Share this @internewscast.com
A revolutionary drug that could significantly slow the progression of type 1 diabetes is currently being trialled by a select group of NHS patients.
The drug Teplizumab, which has already received the green light in the US, is designed to retrain the immune system to halt its attack on pancreatic cells, potentially delaying insulin dependence by an average of three years.
Type 1 diabetes occurs when the immune system mistakenly targets insulin-producing cells in the pancreas, disrupting the body’s ability to regulate blood sugar levels.
Extreme blood sugar levels can lead to severe health complications and even be fatal. As a result, individuals with type 1 diabetes require daily insulin injections to manage their condition.
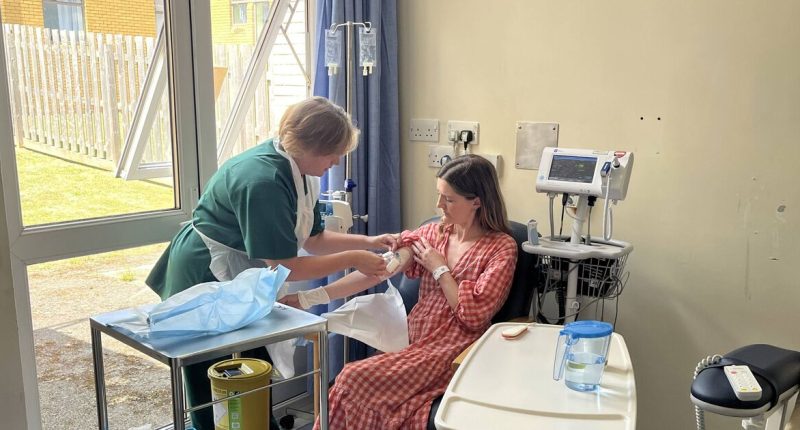
Hannah Robinson, a dentist and mother-of-two from Devon, has become the first adult in the UK to trial the medication, hoping it will postpone the onset of the disease.
She began her treatment at the Royal Devon University Healthcare NHS Foundation Trust after tests during her pregnancy indicated she was in the initial stages of developing type 1 diabetes.
Currently, the drug is being administered to a small number of patients on an individual basis while it undergoes evaluation for broader NHS applications.
The 36-year-old expressed her optimism about the treatment: “For me, this new drug offers more freedom and the chance to focus on my health before I have to start thinking differently and managing life as somebody needing daily insulin.”
She added, “This isn’t just about what I eat or monitoring my glucose, it is also about having more control and not feeling defined by my condition.
“This treatment could potentially pave the way for a future cure for type 1 diabetes, which is incredible. I feel very lucky to be part of this.”
The groundbreaking new drug teplizumab has been revealed to be effective only at the earliest stage of type 1 diabetes.
Dr Nick Thomas, a leading diabetes consultant and academic clinical lecturer at the University of Exeter, enthused: “This new treatment represents a really exciting shift in how we manage type 1 diabetes.
“For the first time ever, we will be able to provide targeted treatment early enough in the process to alter the underlying immune process, aiming to slow down how quickly people need insulin.
“It’s noteworthy that approximately half of all type 1 diabetes cases develop in adulthood, and Hannah will be the first adult in the UK to receive this treatment.
“My hope is that in the future, we may be able to stop people with early type 1 diabetes from needing insulin at all.”
Groundbreaking research conducted by experts at the Royal Devon and the University of Exeter involves using genetics and other testing to identify individuals at high risk of developing type 1 diabetes.
With this innovative approach, there’s the potential that more people could benefit from the drug to delay the onset of type 1.
Dr Lucy Chambers, head of research impact and communications at Diabetes UK, declared: “For people in the early stages of type 1 diabetes, teplizumab offers a groundbreaking opportunity to buy them precious extra years insulin-free.
“Right now, it’s only available in research settings – and while the excitement is real, urgent work is still needed to ensure it reaches everyone who could benefit.
“That means securing a UK licence for teplizumab, establishing national screening programmes to identify people with early-stage type 1 diabetes before symptoms appear, and preparing the NHS to deliver this treatment at scale.
“Diabetes UK is proud to be at the forefront of these efforts – funding pioneering research and working closely with the NHS towards a future where immunotherapies become the first-line treatment for tackling the autoimmune attack at the root of type 1 diabetes.”
Professor Richard Oram, consultant physician at the Royal Devon and professor at the University of Exeter, stated: “Excitingly, teplizumab is the first drug with the potential to delay type 1 diabetes, but needs to be given before clinical diagnosis due to high blood glucose.
“It is really important to find new and improved approaches for identifying individuals at elevated risk.”