Share this @internewscast.com
Health authorities have issued a warning about the weight loss medication Wegovy, indicating it may lead to permanent vision impairment. This potential side effect was initially identified in 2024, when researchers suggested that semaglutide, Wegovy’s active ingredient, could trigger an ‘eye-stroke’. This condition occurs when the blood vessels supplying the eye become obstructed.
There is ongoing debate among experts regarding the prevalence of this problem, medically referred to as non-arteritic anterior ischemic optic neuropathy (NAION), in individuals using the drug for weight loss.
At the time of the initial reports, Novo Nordisk, the pharmaceutical company behind Wegovy, stated that blindness was not a recognized adverse reaction to the drug.
The Medicines and Healthcare products Regulatory Agency (MHRA) in the UK has now confirmed the risk associated with this complication. They advise that individuals using the weight loss treatment should promptly consult a healthcare professional if they experience any changes in their vision.
Semaglutide is also widely used by patients with diabetes in the NHS, where it is marketed under the name Ozempic for managing blood sugar levels.
Semaglutide is also regularly taken by NHS diabetes patients. When given to those with the blood sugar condition, it is known as Ozempic.
In a statement, the Government organisation said: ”Patients taking semaglutide who notice a sudden change in their eyesight, such as sudden impairment to their vision, or if eyesight gets worse very quickly in one or both eyes, should urgently attend eye casualty or A&E.’
Dr Alison Cave, MHRA’s Chief Safety Officer, added: ”While the potential risk of NAION for patients prescribed semaglutide is extremely small, it is important that patients and healthcare professionals are alert to the associated symptoms.’

The MHRA have updated its guidance on the jabs, saying both patients and healthcare professionals need to be aware of the risk

The condition, known as an ‘eye stroke’, occurs when the vessels supplying blood to the optic nerve become blocked
The MHRA also say that Wegovy prescriptions will now come with a safety warning about the risk of blindness.
Officials also said it was vital for healthcare professionals, like GPs, to remain vigilant and enquire about semaglutide use if a patient presents with these symptoms – as privately prescribed semaglutide, for weight loss or diabetes, may not appear on medical records.
SInce semaglutide was authorised in the UK in 2018, there have been three suspected reports of NAION, according to the MHRA.
In the past five years there has been an approximate 10.2millions prescriptions of the drug handed out.
The MHRA says it is now reviewing whether Mounjaro, the other major weight loss jab available in the UK, could also cause blindness.

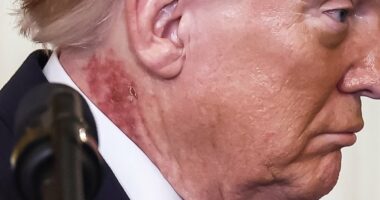
Robbie Williams fears weight loss jabs are ‘making him blind’ after noticing his vision rapidly deteriorating, in a worrying health update (pictured in 2024)
Robbie Williams is just one of the many who expressed concerns that Mounjaro, also known as tirzepatide, was making him blind last year, after noticing his vision rapidly deteriorating.
Last year, the singer, 51, said his eyesight had become increasingly blurry after taking Mounjaro to the point that he was struggling to see people’s faces during live performances.
The former Take That star now has to wear glasses.
Previous studies, published in JAMA Ophthalmology, have also highlighted the link, detailing the case on nine adults in Canada who took either semaglutide or tirzepatide.
Seven were subsequently diagnosed with NAION but most notices their vision improving within days or weeks of stopping the drugs.
There are currently no treatments for NAION, which is thought to affect around one in 10,000 people, and vision often does not improve.
Novo Nordisk, the develop of semaglutide, is currently facing several lawsuits in the US from patients who claim the drug robbed them off their sight.
It comes following a landmark study which suggested weight loss jab users may need to stay on them for life, putting millions at risk of the debilitating condition.
Around 2.5million people are currently thought to be using new GLP–1 drugs in the UK.
Under current NHS rules, only patients with a BMI over 35 and a weight–related health condition – or those with a BMI between 30 and 34.9 referred to specialist services – should receive the drugs.
The arrival of GLP–1 drugs has transformed obesity treatment, offering dramatic weight loss that was largely out of reach with diet and exercise alone.
Concerns have been raised about the risks of the medicines, which can cause nausea, vomiting and diarrhoea, and in rare cases have been linked to pancreatitis, but experts say the benefits far outweigh the risks for most patients.
Novo Nordisk and Eli Lilly, the maker of Mounjaro, were approached for comment.