Share this @internewscast.com

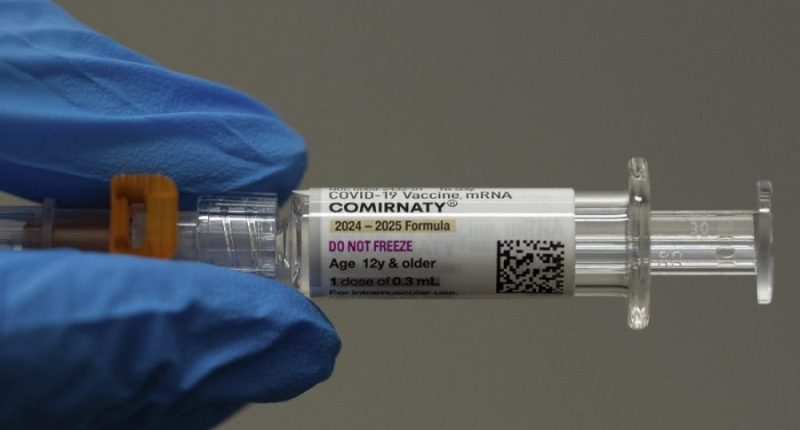
(The Hill) – President Trump’s administration has slammed the brakes on development of messenger RNA (mRNA) vaccines, which proved their lifesaving potential during the COVID-19 pandemic but have come under increasing scrutiny among skeptics of mainstream science.
The vaccines marked a breakthrough in medical technology, drastically reducing the timeline for development of targeted vaccines and even showing promise in cancer research. Trump called mRNA the “gold standard” when he rolled out the first COVID-19 vaccines.
But now they are under assault by Trump’s Health and Human Services (HHS) secretary, longtime anti-vaccine advocate Robert F. Kennedy Jr., and his allies in the “make America healthy again” movement.
HHS in late May canceled $766 million awarded to Moderna through the Biomedical Advanced Research and Development Authority (BARDA) to develop a potential mRNA vaccine for bird flu. HHS spokesperson Andrew Nixon said of the decision, “This is not simply about efficacy it’s about safety, integrity, and trust.”
“The reality is that mRNA technology remains under-tested, and we are not going to spend taxpayer dollars repeating the mistakes of the last administration, which concealed legitimate safety concerns from the public,” he added.
Unlike traditional vaccines that contain fragments or weakened versions of a virus, mRNA vaccines send messenger ribonucleic acid into cells to teach the immune system to recognize proteins connected to virus cells.
According to Joseph Varon, president and chief medical officer of the Independent Medical Alliance (IMA), the concerns for mRNA vaccine skeptics are the expedited timeline and conditions in which the COVID-19 vaccine was approved.
“The biggest concern is that this rushed treatment still remains in use, even under an Emergency Use Authorization in some cases. It needs to be sent back through proper studies and vetting,” Varon told The Hill.
“There needs to be an established database of vaccine injuries that can be accurately quantified with full transparency, without politics or big money pressure influencing the data,” he added. “There’s a growing body of peer-reviewed studies that indicate the spike proteins are causing havoc in certain recipients.”
The IMA, previously known as Front Line COVID-19 Critical Care Alliance, launched a campaign in support of Kennedy’s nomination to be HHS secretary. The group drew controversy when its founders promoted ivermectin as a “miracle drug” for COVID-19.
Myocarditis, or inflammation of the heart muscle, is of particular concern among mRNA vaccine opponents. An analysis published in 2022 found that myocarditis occurred in about 31.2 cases per 1 million second doses of the Moderna COVID-19 vaccine, or 0.003 percent.
Rates of myocarditis are significantly higher among people with COVID-19 infections than immunizations, however.
The development of the COVID-19 mRNA vaccine was aided by the Trump administration’s Operation Warp Speed, something Trump has boasted about at numerous points.
In remarks in December 2020, the same month the first COVID-19 vaccines were deployed, Trump praised Operation Warp Speed’s ability to develop a SARS-CoV-2 vaccine at a “breakneck speed,” adding “the gold standard vaccine has been done in less than nine months.”
Though research on mRNA and its potential uses has been ongoing for decades, use of the vaccines in humans is relatively new.
The first mRNA vaccine candidate tested in humans was for rabies in 2013, but it wouldn’t be until the COVID-19 pandemic in 2020 that this technology would achieve commercial viability and widespread use.
To Kennedy, this is too much of a coincidence.
In his book “The Wuhan Cover-Up: And the Terrifying Bioweapons Arms Race,” published in 2023, Kennedy suggests it was more than just happenstance that allowed for the deployment of Moderna’s mRNA technology to coincide with the global pandemic.
He pointed to a meeting attended by Dr. Anthony Fauci and former BARDA Director Rick Bright in which the officials met with virologists months before the COVID-19 pandemic broke out. The fact that attendees discussed the need for innovation in influenza research and vaccines became a point of conspiracy online.
Kennedy noted that Bright called for something “completely disruptive, that’s not beholden to bureaucratic strings and processes,” writing, “[Bright] hinted that only a global crisis like a pandemic could induce government and industry to commit the billions of dollars necessary to create a new generation of ‘plug-and-play’ mRNA vaccines.”
In the conversation Kennedy references, Bright was discussing the challenges of making influenza research “sexy” for Ph.D. and postdoctoral students, surmising that something “completely disruptive” would be needed to incite excitement in the field.
Proponents of the mRNA technology point to its deployment in the COVID-19 pandemic and its role in reducing transmission as evidence of safety and efficacy.
“In the U.S., you know, we have a lot of real-world experience now with huge numbers of doses given. We have been following for significant or serious adverse events for a long time, and the rate of those serious adverse events is on par with what we see for other vaccines,” E. John Wherry, director of the Institute for Immunology and Immune Health at the University of Pennsylvania, said of mRNA vaccines.
As Wherry notes, all vaccines have adverse side effects, but their safety compared with the diseases they inoculate against is “incontrovertible.” And while critics take issue with the speed at which mRNA vaccines are developed, Wherry said this difference is precisely what sets these shots apart from older generations.
“mRNA vaccines have a couple of key benefits or features that really stand out compared to other vaccine platforms. One, they can be generated very, very quickly. So, you can adapt to changes very rapidly. This is much easier with an mRNA vaccine, where the COVID strain or flu strain mutates and changes from year to year,” Wherry said.
“The second is that it’s relatively simple. So, we don’t have to worry about, you know, impurities in, you know, an egg-grown vaccine or a cell-based grown vaccine synthesizing mRNA. There are very few components that go into it, and it can be synthesized with high purity.”
Such mRNA vaccines are also potentially applicable for numerous conditions or even multiple different strains of the same virus, giving it more flexibility than traditional vaccinations.
“So, there are substantial benefits in flexibility, in speed, in simplicity of manufacturing and that probably also affects the cost of goods at the end of the day, which is perhaps a last advantage,” Wherry added.