Share this @internewscast.com
A first-of-its-kind treatment to slow the symptoms of early Alzheimer’s disease has been approved for use in Australia.
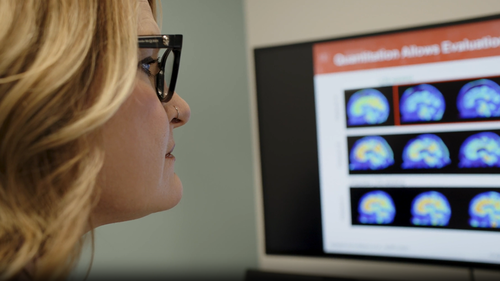
The Therapeutic Goods Administration has approved the pharmaceutical company Eli Lilly’s medication, Kisunla (donanemab), for individuals experiencing early symptomatic Alzheimer’s, particularly those who exhibit amyloid plaque, or protein clusters, in the brain.
A build-up of plaque causes issues associated with the neurodegenerative disease, like memory loss and impaired thinking.

The drug is administered through a monthly infusion over a maximum of 18 months and works to clear plaque from the brain.
According to Eli Lilly, Kisunla is the first novel therapy for early-stage Alzheimer’s in 25 years and the first in Australia aimed at addressing the root cause of the condition.
An estimated 600,000 Australians currently live with Alzheimer’s with about 450,000 of those able to be tested to assess their eligibility.
This has been hailed as a breakthrough by the researcher who led the clinical trials in Australia. 
Professor Michael Woodward, Head of Dementia Research at Austin Health, expressed, “As clinicians, we’ve been anticipating the opportunity to provide patients with a treatment like this for a very long time.”

“There is an important window of opportunity when it is possible to detect, diagnose and treat Alzheimer’s disease with the goal that patients can maintain their independence for longer.
“The availability of donanemab is a major development at a time when more and more Australians and their families are being impacted by this insidious disease.”
The drug is not listed on the Pharmaceutical Benefits Scheme (PBS), meaning that it would cost patients $4700 per session or $84,600 over the recommended 18 months.
An application to include the drug on the PBS will be reviewed in July.
Donanemab is approved in the United States, Japan, China, the United Kingdom, United Arab Emirates, Qatar, Kuwait, Bahrain, Singapore, Taiwan, Brazil, Mexico and Australia.