Share this @internewscast.com
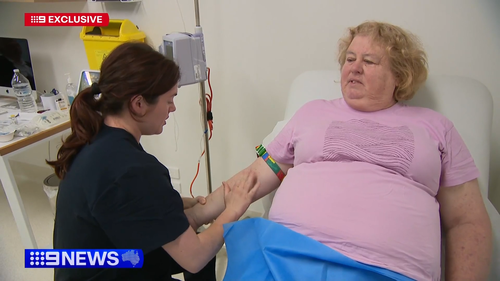
A 61-year-old grandmother from New South Wales is among the first Australians to receive a newly approved medication for early Alzheimer’s disease.
The drug, called Kisunla or Donanemab, is the first new medicine for early Alzheimer’s disease in 25 years.
“Donanemab and the anti-amyloid class of medications is really a breakthrough, a new age in Alzheimer’s treatment,” neurologist Dr Rowena Mobbs said.

“We now have something to slow the condition after 100 years of research.”
Jenny Quiring, whose nursing career was curtailed by early symptoms of Alzheimer’s, has just received the drug.Â
“It’s probably the first little bit of hope,” husband James Quiring said.
The treatment will give Jenny a chance to spend more quality time with her husband of 41 years, their seven children and 15 grandchildren.
“We’d like to see them grow up and be happy,” she said.

The half-hour monthly infusions will be costly, with the required scans and an 18-month course of treatment costing up to $100,000.
A government advisory panel is anticipated to make a decision soon on the inclusion of Donanemab in the Pharmaceutical Benefits Scheme (PBS).
“It should be on the PBS, it’s definitely worth it,” Jenny said.
The producer of the medication has published extended data showing that the advantages of the therapy grow over a period of three years. The research also indicates that starting treatment earlier can lower the risk of disease advancement by 27 percent compared to postponing it.