Share this @internewscast.com
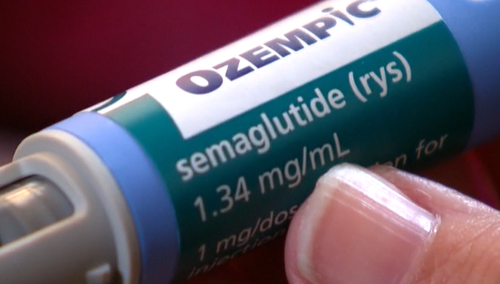
Australia’s regulatory body for medications has issued a new advisory concerning popular injectable drugs used for treating diabetes and weight loss, including Ozempic.
On the latest update, the Therapeutic Goods Administration has expanded its guidance for the class of GLP-1 receptor agonists. This includes alerts about the possible onset of suicidal thoughts and additional contraception warnings for a specific brand.
This revision in self-harm advisories comes in the wake of international observations pointing to a “potential risk of suicidal thoughts or behaviors.”

It is advised that none of these drugs should be used by individuals who are pregnant.
Originally developed for type 2 diabetes management, these injectable medications have surged in popularity due to their appetite-reducing effects.
They are prescribed for obesity management and are also widely used by individuals looking to lose weight.
But researchers have also raised several health concerns, such as arthritis and stomach issues, while noting the potential for health benefits for overweight people stemming from weight loss.
Potential long-term side effects of the new drugs are yet to be fully understood.
Readers seeking support can contact Lifeline on 13 11 14 or beyond blue on 1300 22 4636.