Share this @internewscast.com
Babies could soon be born without biological mothers – as scientists have come up with a way to create human eggs from skin cells.
The technique opens the possibility for DNA from a man’s skin cells being placed indside a donor egg, before being fertilised by another man.
In theory, this could allow two men to have a baby, withou any DNA from a woman.
The breakthrough could also help women experiencing problems with their eggs to have their own genetic children.
Although additional research is required to confirm safety and effectiveness before moving to clinical trials, specialists have hailed the development as a ‘significant breakthrough.’
‘Many women are unable to start a family because they have lost their eggs due to various reasons, such as after undergoing cancer treatment,’ explained Professor Richard Anderson, Deputy Director of MRC Centre for Reproductive Health at the University of Edinburgh, who was not part of the study.
‘The capability to create new eggs would represent a major breakthrough. The study demonstrates that genetic material from skin cells can be utilized to produce a cell similar to an egg, containing the correct chromosome count needed for fertilization and early embryo development.’
‘There will be very important safety concerns but this study is a step towards helping many women have their own genetic children.’

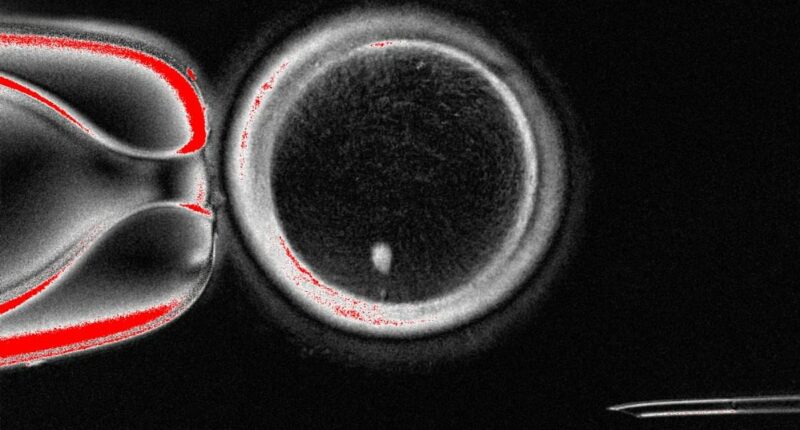
Experts from Oregon Health & Science University have created fertilizable eggs from human skin cells for the very first time
For some couples struggling to conceive, in virto fertilization (IVF) can be an option.
This treatment sees the eggs fertilized by sperm in a lab, and the resulting embryo then placed in the woman’s uterus.
However, if there’s a problem with the egg itself, IVF can be ineffective.
Previous studies have suggested that a method called ‘somatic cell transfer’ could be an alternative approach.
This process involves transferring the nucleus from one of the patient’s own somatic cells, like skin cells, into a donor egg cell that has had its nucleus removed, allowing the cell to transform into a viable egg.
Nonetheless, while normal eggs possess half the typical number of chromosomes (one set of 23), cells derived from skin cells offer two sets of chromosomes (46).
Without intervention, this would cause the differentiated eggs to have an extra set of chromosomes.
So far, a method to remove this extra set has been developed and tested in mice – but is yet to be tried in humans.

For some couples facing challenges with conception, in vitro fertilization (IVF) may be a viable option. In this treatment, eggs are fertilized by sperm in a laboratory setting, with the subsequent embryo being placed in the woman’s uterus (illustrative image).
In their new study, the team resolved this issue by inducing a process they’ve named ‘mitomeiosis’.
‘[Mitomeiosis] mimics natural cell division and causes one set of chromosomes to be discarded, leaving a functional gamete,’ the researchers explained in a statement.
During tests, the researchers were able to produce 82 functional eggs using this process, which were then fertilised in a lab.
Approximately nine per cent went on to develop the the blastocyst stage of embryo development.
However, the researchers did not culture the blastocysts beyond this point, which coincided with the time at which they would usually be transferred to the uterus in IVF treatment.
While the findings raise the tantalising possibility of women with problems with their eggs having their own genetic children, the experts note several limitations with their study.
Importantly, the vast majority (91 per cent) did not progress beyond fertilisation.
What’s more, several of the blastocysts were found to contain chromosomal abnormalities.
Regardless, experts have called the research an ‘exciting proof of concept’.
‘This breakthrough, called mitomeiosis, is an exciting proof of concept,’ said Professor Ying Cheong, a professor of reproductive medicine at the University of Southampton, who was not involved in the research.
‘In practice, clinicians are seeing more and more people who cannot use their own eggs, often because of age or medical conditions.
‘While this is still very early laboratory work, in the future it could transform how we understand infertility and miscarriage, and perhaps one day open the door to creating egg– or sperm–like cells for those who have no other options.’