Share this @internewscast.com
The FDA announced a radical shift in its Covid vaccination policy.
Annual updated Covid shots will no longer be automatically approved, the agency said in a press conference Tuesday.
The FDA now requires companies to perform clinical trials to demonstrate that Covid vaccines remain safe and effective before they gain approval for use in healthy adults and children.
The agency has been approving annually updated vaccines, but this new requirement may increase costs for vaccine manufacturers, potentially reducing the volume of vaccines produced and limiting access.
Nevertheless, for individuals over 65 and those with weakened immune systems or existing health conditions, the FDA will maintain a simplified approval process.
The guidance was published in an editorial in the New England Journal of Medicine, authored by FDA head Dr Marty Makary and the agency’s head of vaccines Dr Vinay Prasad.
Dr Makary says the change is needed, adding there is no clear benefit from vaccinating millions of healthy people against Covid every year.
He also said the policy has fostered public distrust and led many to forgo vital vaccinations, such as those against measles — with the US currently battling its biggest outbreak in two decades.

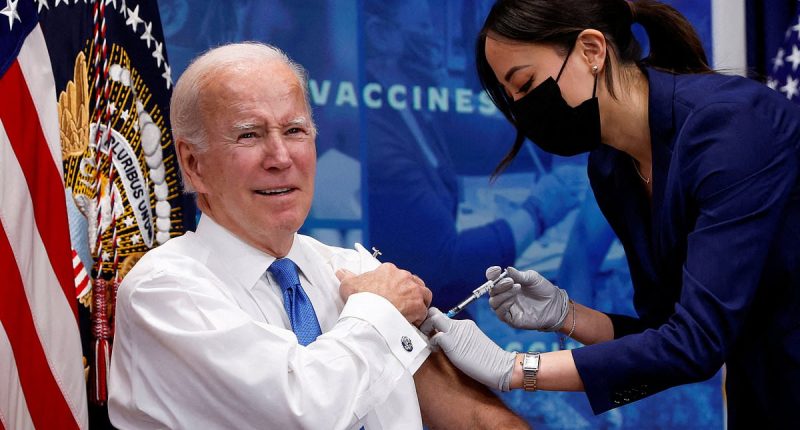
The FDA has revealed an overhaul to its Covid vaccine policy. Pictured above is Biden getting an updated Covid vaccine in 2022
Dr Prasad said the change will now be considered by the FDA’s vaccine advisory committee.
The new recommendation will likely affect people who are seeking an updated Covid vaccine in fall 2025, but Dr Prasad insisted most will still be able to get it.
As well as offering it to the 57.8million who are over 65 years old, he said the shot would also be available to the estimated 100 to 200million people who have an underlying condition, putting them at higher risk of Covid.
The CDC maintains a list of at least 20 conditions that raise the risk of suffering from severe Covid, including conditions like obesity and physical inactivity.
Dr Prasad said: ‘We have launched down this multi-year campaign of booster after booster after booster.
‘[But] we do not have the gold standard science to support this for average-risk, low risk, Americans.’
About 70 percent of Americans had received at least one dose of the Covid vaccine by May 2023.
But now most people are forgoing boosters, with data showing that just 23 percent came forward for them last year — despite an FDA-backed recommendation that everyone aged six months and over should get the dose.
The US has been out of step with other western nations on boosters, with most — including the UK, Canada and Australia — now only recommending them for older adults.
The proposal is set to be considered by the FDA’s Vaccines and Related Biological Products Advisory Committee at its meeting on Thursday, which will make a recommendation.
The FDA commissioner, Dr Makary, will then make a final call on whether to change the vaccine guidance.

Dr Vinay Prasad, who heads up the FDA’s research arm, is pictured above during the press conference

The conference was followed by a sit-down conversation between Dr Prasad and the FDA commissioner Dr Marty Makary (pictured)
The CDC’s vaccine body — the Advisory Committee on Immunization Practices — may also make a recommendation on who should get a Covid booster vaccine.
As for new approval, Dr Prasad set out new requirements for vaccine manufacturers seeking to have Covid vaccines approved for under-65s.
He said there was too little data to recommend the shots for this group, instead saying that new randomized placebo-controlled trials were needed.
Former FDA commissioner Dr Robert Califf supports this view, writing in JAMA in January: ‘Covid vaccine uptake is now low enough that large [randomized clinical trials] are feasible to evaluate the efficacy and safety of new updated boosters.’
A randomized clinical trial is a study where participants are assigned to at least two groups, with only one receiving the treatment or factor being tested, while the other receives a placebo.
These studies are widely considered to be the gold standard for evaluating the safety and efficacy of any new vaccines.
Dr Prasad said the FDA would be interested in studies investigating the effectiveness of Covid boosters among people aged 50 to 65 years old.
He added: ‘America is deeply divided on the policy of repeat Covid-19 vaccine doses or boosters.

The above shows current vaccine booster recommendations in other western countries. The US is very much out of step with other nations

This shows the underlying conditions that put someone at risk of disease. The FDA is proposing that anyone with these conditions should still be able to get a booster vaccine

Updated Covid vaccines will now only be offered to adults aged 65 years and older
‘There are some Americans out there who are worried that the FDA has not fully documented and interrogated the safety harms of these products and they are categorically opposed to these products.
‘There are also some Americans, we also have to recognize, who are desperate for additional protection, and they demand these products.
‘But the truth is that most doctors, and most of the public, are entirely uncertain [about whether they need to get a Covid booster].’
Dr Noel Brewer, a public health expert at the University of North Carolina, Chapel Hill, and who sits on the CDC’s vaccine recommendation committee, told CNN he supported the change.
‘The proposed policy moves the US into line with other countries. This global view of public health is a welcome development,’ he said.
Dr Paul Offit, a pediatrician at the Children’s Hospital of Philadelphia and a member of the FDA’s vaccines advisory group, disagreed, however.
He said: ‘We have been using an evidence-based approach to Covid vaccination, but they kind of swoop in and believe that for the first time, we’re going to get, as they say, “gold standard” data, robust data, for the first time, because, according to them, we don’t have that, but we do have that.
‘That’s why we’ve made good decisions about the vaccines. That’s why that vaccine is remarkably safe. I mean, the mRNA vaccines are remarkably safe.’