Share this @internewscast.com
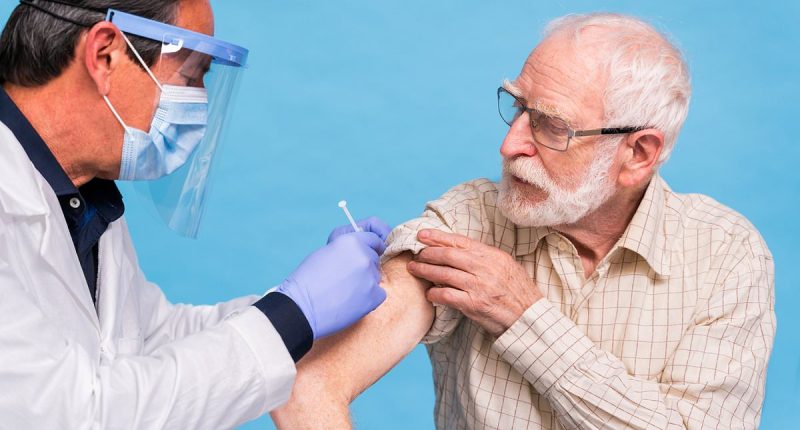
UK vaccine chiefs have suspended a newly approved jab from being given to people aged over 65, after reports of two deaths and 21 severe reactions.
The vaccine protects against the potentially fatal Chikungunya virus, which is typically found in the subtropical regions of the Americas, Africa, Southeast Asia, India, the Pacific Region and increasingly in Europe.
Although the virus isn’t found in the UK, Britons can become infected while overseas and fall ill when they return home.
While the jab, called IXCHIQ, is approved in the UK it has yet to be rolled out, so there are no immediate safety concerns, British regulators said.
The suspension follows global reports of 23 serious adverse effects in older people, including two deaths.
It followed two fatalities in the French island La Réunion—in the Indian Ocean—where a vaccination campaign is underway following a recent Chikungunya outbreak.
One death involved an 84-year-old man who developed encephalitis—a swelling of the brain—after having an adverse reaction to the vaccine.
The other was a 77-year-old man living with Parkinson’s disease who reportedly began finding swallowing increasingly difficult after the jab.

The vaccine is for the Chikungunya virus, which isn’t found in the UK, but Britons can catch it from overseas and fall ill when they return home
This is believed to have triggered aspiration pneumonia, a serious infection caused by food, saliva and/or vomit entering the lungs rather than the stomach.
The European Medicines Agency, who are reviewing the chikungunya vaccine following the reports, has not yet revealed the time between the dead men receiving the jabs and their deaths.
There are no changes in the recommendations for vaccination with IXCHIQ for people aged between 18 and 64.
The vaccine is currently not approved for use in individuals with a weakened immune system as a result of disease or medical therapy.
The decision by the Commission on Human Medicines (CHM) is a precautionary measure until a further safety review has been concluded.
The Medicines and Healthcare products Regulatory Agency (MHRA) is working with the vaccine manufacturer Valneva.
The majority of people infected with Chikungunya develop a sudden fever and severe pain in multiple joints.
Other symptoms may include headache, muscle pain, joint swelling, or rash.

UK vaccine chiefs have suspended a newly approved jab for over 65s after reports of two deaths and 21 severe reactions
The virus is spread to humans by bites from infected mosquitoes, but cannot be passed from person-to-person.
Symptoms typically resolve within 7 to 10 days, and most patients make a full recovery.
However, in some cases the joint pain and arthritis may persist for several months or even years.
Occasional cases of eye, neurological and heart complications have been reported, as well as gastrointestinal complaints.
A small number of people may develop a severe version of the disease, which can lead to multiple organ failure and death.
Severe symptoms and deaths are rare and usually occur in young babies or elderly people with coexisting health problems.
There is no specific antiviral treatment for Chikungunya but medications (such as paracetamol) for fever and pain can be used to alleviate these symptoms.
There are currently two vaccinations which protect against the virus; IXCHIQ for those aged between 18 and 64 and Vimkunya for those 12 years and older.
A recent outbreak in La Réunion has seen over 47,500 cases of the virus, with 12 fatalities.
In 2024 there were 112 Chikungunya cases reported in England, Wales and Northern Ireland from travellers arriving to or returning to Britain—this was nearly one-and-a-half times the level recorded in the previous year.