Share this @internewscast.com
U.S. regulators have given the green light to updated COVID-19 vaccines on Wednesday, although they’ve restricted their use for a large portion of the American population and have discontinued one of the vaccines available for young children.
The newly updated vaccines from Pfizer, Moderna, and Novavax have been approved for all seniors. However, the Food and Drug Administration has limited the availability of these vaccines for younger adults and children to those who have at least one high-risk health condition, like asthma or obesity. This policy introduces new barriers for millions of Americans who now need to prove they are at risk, as well as for others who wish to get vaccinated but find they no longer meet the criteria.
Moreover, the FDA has revoked Pfizer’s vaccine’s emergency use authorization for children under 5, meaning it will no longer be an option for that age group.
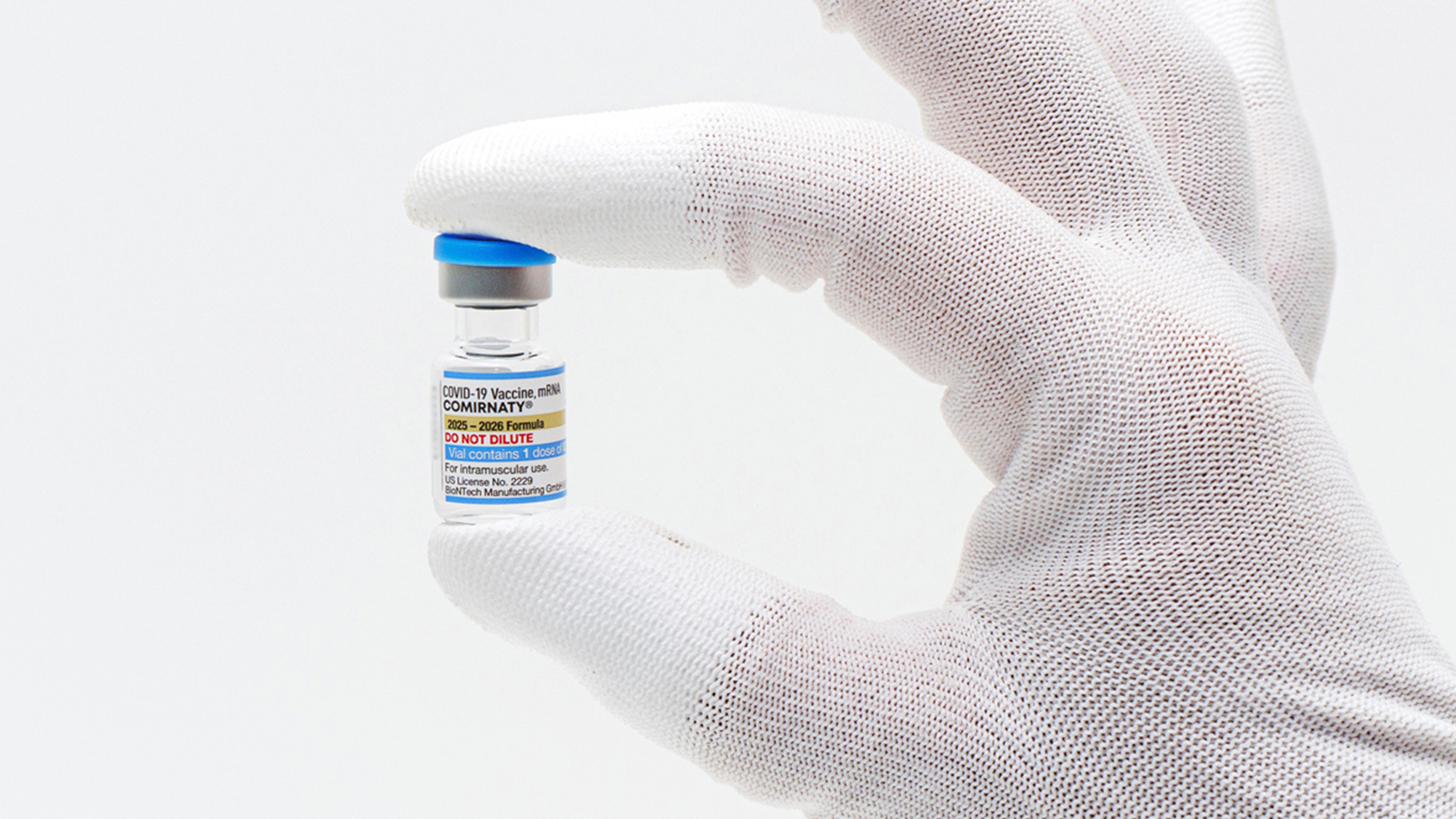
This photo provided by Pfizer in August 2025 shows a vial of the updated COVID-19 vaccine Comirnaty.
Pfizer via AP
Parents can still opt for Moderna’s vaccines, the other set of mRNA vaccines, which remain fully approved by the FDA for children as young as 6 months old. However, Moderna’s Spikevax is only approved for children with at least one serious health issue.
The revised vaccines are designed to combat a more recent strain of the ever-mutating virus and are prepared to be distributed immediately. However, it may take several days or even weeks before many Americans can discover if they qualify for the updated vaccine, as access will rely on decisions from federal health consultants, private insurance providers, pharmacies, and state regulations.
These new limitations, anticipated by FDA officials back in May, represent a shift from the former U.S. policy which advocated for an annual COVID-19 vaccine for all individuals aged 6 months and older.
The approach reflects heightened skepticism about the ongoing risks of COVID-19 and the need for yearly booster shots from Health Secretary Robert F. Kennedy Jr. and FDA Commissioner Marty Makary, who were both outspoken critics of wide-scale vaccinations.
“The American people demanded science, safety, and common sense. This framework delivers all three,” Kennedy wrote on social media.
Kenned said Novavax’s shot is only open to people 12 or older, not younger children – and under the same risk-based restrictions as are now in place for the Moderna and Pfizer options. It’s the nation’s only protein-based COVID-19 vaccine.
Some medical groups, including the American Academy of Pediatrics, have objected to the new limits, saying they may block vaccine access for families who want to protect their children. Last week, the group offered its own recommendations for kids, saying annual COVID shots are strongly recommended for children ages 6 months to 2 years and advised for older children.
That differs from the latest guidance under Kennedy, which doesn’t recommend the shots for healthy children of any age, but says kids may get the shots in consultation with physicians.
Many countries have scaled back COVID-19 vaccinations in recent years, and some U.S. experts say the change makes sense here, given that nearly all Americans have some protection from previous vaccination or infection.
But implementing the shift presents a number of logistical problems.
Insurers typically base their vaccine coverage decisions on the recommendations of a panel of advisers to Centers for Disease Control and Prevention, but some say they will also look to medical professional groups, including the American Medical Association.
Earlier this year, Kennedy dissolved the CDC panel and replaced its members with a number of doctors and researchers who have repeatedly questioned the safety of commonly used vaccines and ingredients. The panel is expected to meet in September, but no specific date has been set and no agenda released.
Depending on the panel’s advice, Americans underage 65 could be expected to provide documentation of a serious medical condition before they can get a shot. Complicating the rollout is the fact that pharmacists – who administer most COVID vaccines in the U.S. – typically aren’t expected to collect that kind of information. And laws governing their ability to administer routine vaccinations vary by state, where pharmacists are licensed.
Nearly half states limit vaccinations by pharmacists to those recommended by the CDC panel.
Access could also be complicated for healthy adults and children who are interested in getting a shot for extra protection.
If the latest vaccines aren’t covered by their insurance, those patients could be required to pay $150 or more out of pocket if they want one. They might also have to find a doctor or other health professional willing to give the shot “off label,” or outside the use listed on the FDA label. Pharmacists may be reluctant to give vaccines off-label.
The updated shots from Pfizer, Moderna and Novavax target a coronavirus subtype named LP. 8.1, a recently dominant version of the virus that is closely related to some newly emerging cousins.
All three shots were initially made available under the FDA’s emergency use authorization, an expedited process to quickly review vaccines and other countermeasures during the pandemic.
Moderna received full FDA approval in July for children down to 6 months and says it should have enough supply to meet U.S. demand.
In addition to revoking emergency use of Pfizer’s vaccine, the FDA also pulled authorization for several other therapies from the pandemic years, including convalescent plasma, which was used to treat hospitalized COVID-19 patients before the first antiviral drugs became available.
COVID-19 vaccines do a good job preventing severe disease, hospitalization and death, which remain a bigger risk for seniors and people with underlying health factors, including heart disease, lung disease and cancer.
Preliminary data from the CDC estimates 47,500 Americans died from COVID-related causes last year. In at least two-thirds of those cases, COVID-19 was listed as the underlying cause of death. For the rest, COVID-19 was a contributing factor.
___
AP Medical Writer Lauran Neergaard contributed to this story.
Copyright © 2025 by The Associated Press. All Rights Reserved.