Share this @internewscast.com

A baby born with a rare and dangerous genetic disease is growing and thriving after getting an experimental gene editing treatment made just for him.
In a newly published study, researchers have highlighted the case of a patient who is among the first to receive successful treatment through a personalized therapy. This approach aims to correct a minor yet crucial genetic error that proves fatal for half of the infants affected. Although it might take some time for such tailored treatments to be widely accessible, medical professionals are optimistic that one day this technology could benefit the millions with rare conditions often overlooked despite advances in genetic medicine.
“This marks an initial step towards utilizing gene editing therapies to address a broad range of rare genetic disorders that currently lack definitive medical treatments,” stated Dr. Kiran Musunuru, a gene editing specialist from the University of Pennsylvania and co-author of the study, which was released on Thursday in the New England Journal of Medicine.
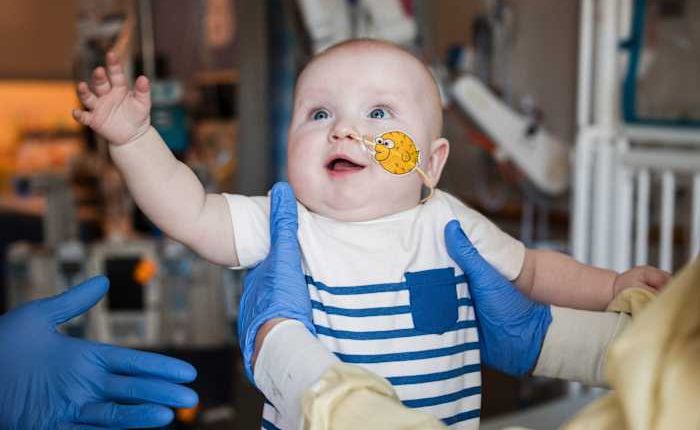
KJ Muldoon from Clifton Heights, Pennsylvania, is the infant in focus, and he is one among 350 million people globally suffering from rare diseases, the majority of which are genetic. Shortly after his birth, he was found to have severe CPS1 deficiency, a condition some experts believe affects roughly one in a million newborns. These infants are deficient in an enzyme necessary for removing ammonia from their bodies, leading to its accumulation in the blood, which can become toxic. A liver transplant could be a potential solution for some of these cases.
Knowing KJ’s odds, parents Kyle and Nicole Muldoon, both 34, worried they could lose him.
“We were, like, you know, weighing all the options, asking all the questions for either the liver transplant, which is invasive, or something that’s never been done before,” Nicole said.
“We prayed, we talked to people, we gathered information, and we eventually decided that this was the way we were going to go,” her husband added.
Within six months, the team at Children’s Hospital of Philadelphia and Penn Medicine, along with their partners, created a therapy designed to correct KJ’s faulty gene. They used CRISPR, the gene editing tool that won its inventors the Nobel Prize in 2020. Instead of cutting the DNA strand like the first CRISPR approaches, doctors employed a technique that flips the mutated DNA “letter” — also known as a base — to the correct type. Known as “base editing,” it reduces the risk of unintended genetic changes.
It’s “very exciting” that the team created the therapy so quickly, said gene therapy researcher Senthil Bhoopalan at St. Jude Children’s Research Hospital in Memphis, who wasn’t involved in the study. “This really sets the pace and the benchmark for such approaches.”
In February, KJ got his first IV infusion with the gene editing therapy, delivered through tiny fatty droplets called lipid nanoparticles that are taken up by liver cells.
While the room was abuzz with excitement that day, “he slept through the entire thing,” recalled study author Dr. Rebecca Ahrens-Nicklas, a gene therapy expert at CHOP.
After follow-up doses in March and April, KJ has been able to eat more normally and has recovered well from illnesses like colds, which can strain the body and exacerbate symptoms of CPS1. The 9 ½-month old also takes less medication.
Considering his poor prognosis earlier, “any time we see even the smallest milestone that he’s meeting – like a little wave or rolling over – that’s a big moment for us,” his mother said.
Still, researchers caution that it’s only been a few months. They’ll need to watch him for years.
“We’re still very much in the early stages of understanding what this medication may have done for KJ,” Ahrens-Nicklas said. “But every day, he’s showing us signs that he’s growing and thriving.”
Researchers hope what they learn from KJ will help other rare disease patients.
Gene therapies, which can be extremely expensive to develop, generally target more common disorders in part for simple financial reasons: more patients mean potentially more sales, which can help pay the development costs and generate more profit. The first CRISPR therapy approved by the U.S. Food and Drug Administration, for example, treats sickle cell disease, a painful blood disorder affecting millions worldwide.
Musunuru said his team’s work — funded in part by the National Institutes of Health — showed that creating a custom treatment doesn’t have to be prohibitively expensive. The cost was “not far off” from the $800,000-plus for an average liver transplant and related care, he said.
“As we get better and better at making these therapies and shorten the time frame even more, economies of scale will kick in and I would expect the costs to come down,” Musunuru said.
Scientists also won’t have to redo all the initial work every time they create a customized therapy, Bhoopalan said, so this research “sets the stage” for treating other rare conditions.
Carlos Moraes, a neurology professor at the University of Miami who wasn’t involved with the study, said research like this opens the door to more advances.
“Once someone comes with a breakthrough like this, it will take no time” for other teams to apply the lessons and move forward, he said. “There are barriers, but I predict that they are going to be crossed in the next five to 10 years. Then the whole field will move as a block because we’re pretty much ready.”
———-
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group and the Robert Wood Johnson Foundation. The AP is solely responsible for all content.
Copyright 2025 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed without permission.