Share this @internewscast.com
Some individuals with elevated cholesterol levels find that lifestyle adjustments and regular statin use fall short in achieving their desired cholesterol goals.
For these individuals, an innovative experimental medication might present a promising alternative.
Recent findings from a Phase 3 trial revealed that incorporating the investigational drug enlicitide into a standard cholesterol-lowering regimen, such as statins, led to as much as a 60% reduction in LDL, or “bad” cholesterol, over a 24-week period. This data was shared in a recent news release.

These results were compared to those of participants who received a placebo alongside their usual cholesterol-lowering treatments.
All individuals involved in the study had elevated LDL cholesterol levels and either had experienced a major cardiovascular event previously or were at heightened risk for one.
The findings, which are yet to appear in a peer-reviewed journal, were unveiled yesterday at the American Heart Association’s Scientific Sessions.
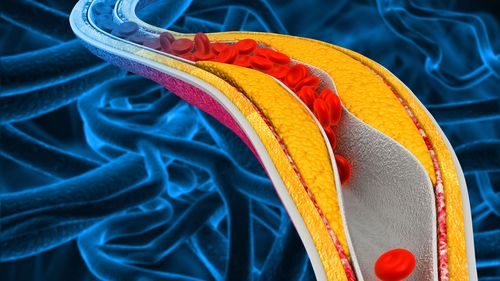
Having high cholesterol in the blood can lead to a buildup called “plaque” on the walls of the arteries, raising the risk of cardiovascular disease.
Heart disease is the leading cause of death in the US – it’s estimated that one person dies every 34 seconds from cardiovascular disease in the US.
Merck, the pharmaceutical company behind enlicitide, plans to apply for US Food and Drug Administration approval of enlicitide early next year, Dr Puja Banka, the company’s associate vice president of clinical research and global clinical development, said.
“We wanted to show what enlicitide can deliver on top of statins, because we know that 70 per cent of patients that are treated with lipid-lowering therapies still don’t reach their guideline-directed goals,” Banka said about the new data.
“So, the idea is really to show what we can achieve with enlicitide on top of statins.”
When statins alone are not enough
The new Phase 3 trial included 2912 adults, with an average age of 63, who either had a previous heart attack or stroke or were at intermediate or high risk of a heart attack or stroke within the next 10 years.
Data were collected across 14 countries between August 2023 and July 2025.
All of the adults in the study had elevated LDL cholesterol levels despite being on a stable course of lipid-lowering therapy like statins for 30 days or more.
About 97 per cent of them were taking statins and 26 per cent were also taking another type of medication to help lower cholesterol levels â and they all continued these therapies during the study.
But as an additional treatment, some of the adults were administered a once-daily pill of enlicitide and others were given a placebo.
Enlicitide is a type of cholesterol-lowering drug known as a PCSK9 inhibitor, which works by helping to clear “bad” LDL cholesterol out of the bloodstream.
On the other hand, statins lower LDL cholesterol by blocking an enzyme in the liver, which then forces the liver to remove more LDL cholesterol from the body.
“Thus, it might be expected that adding enlicitide that works by a different mechanism to statins could further reduce LDL cholesterol,” Dr Kristin Newby, a cardiologist and professor at Duke University School of Medicine, who was not involved in the study, said in an email.
The researchers found that after 24 weeks, the adults taking enlicitide not only saw up to a 60 per cent reduction in LDL cholesterol, but reductions were sustained through 52 weeks.
The adults taking enlicitide also showed a 53 per cent reduction in a combination of all other cholesterol except for “good” HDL cholesterol; a 50 per cent decrease in ApoB, a protein that helps carry fat and various “bad” types of cholesterol throughout the body; and a 28 per cent drop in a type of fat in the body known as lipoprotein(a).

As for the safety of the drug, about 10 per cent of the adults taking enlicitide experienced serious adverse events during the study and a similar share, about 12 per cent, of those taking a placebo experienced adverse events in the study.
“Ultimately, the side effects were balanced between the enlicitide arm and the placebo arm,” Banka said.
“There wasn’t a particular adverse event that stands out as imbalanced between the two.”
Some of the adverse events reported in the trial were gastrointestinal disorders and certain infections, but the occurrence of these events was similar across both groups.
“This provides some reasonable assurance of no major or high frequency adverse events or side effects,” Newby, who is an expert with the American Heart Association, said.
“However, similar to other new therapeutics, larger outcomes studies and post-marketing surveillance will be needed to identify any low-frequency adverse effects that might occur,” Newby said.
“The major benefit that is clear from this study is that enlicitide is very efficacious at lowering LDL cholesterol (by about 55 per cent) compared with placebo on a background of statin therapy, and secondarily, it helps more people reach their LDL cholesterol treatment goal than with statins alone.”
Expanding options for patients
For people with high LDL cholesterol who may not respond sufficiently to commonly used treatments like statins alone, there is already the option to take injectable cholesterol-lowering PCSK9 inhibitors that are currently on the market.
But a key difference between the PCSK9 inhibitors already on the market and the experimental enlicitide medication is that those currently on the market are administered as an injection.
“Whereas enlicitide is an oral medication taken daily. Thus, offering an option in route and frequency of administration that may be preferable to some patients,” Newby said.
Banka described enlicitide as the “first oral PCSK9 inhibitor to complete Phase 3 trials.”
One new study has demonstrated that a PCSK9 inhibitor already on the market can reduce major cardiovascular disease events in adults with no prior heart attack or stroke.
Adding the injectable PCSK9 inhibitor evolocumab â sold as Repatha â to a cholesterol-lowering treatment regimen was found to reduce the risk of a first-time major cardiovascular event among adults with atherosclerotic cardiovascular disease or diabetes, according to a separate study presented Saturday at the American Heart Association’s Scientific Sessions.

The trial was conducted across 33 countries between June 2019 and November 2021, and it included 12,257 adults, with an average age of 66.
The adults were randomly separated into two groups.
One group received evolocumab injections under the skin every two weeks while the other group received injections of a placebo every two weeks.
The data showed that, after an average of 4.6 years, adults in the evolocumab group saw a 25 per cent reduced risk of coronary heart disease deaths, heart attack or ischemic stroke, compared with those in the placebo group.
In the United States, evolocumab injection has already been approved by the FDA, and has been approved in Australia since 2022 and is part of the Pharmaceutical Benefits Scheme.
The medication is indicated to reduce the risk of heart attack and stroke in adults with established cardiovascular disease as well as to be used as an “add-on” treatment for certain forms of high cholesterol.
The latest trial results represent “the first demonstration of improved cardiovascular outcomes with a PCSK9 inhibitor, or any non-statin for that matter, in patients without a previous heart attack or stroke who are already being treated with a high-intensity lipid-lowering regimen,” lead study author Dr Erin Bohula said in a news release on Saturday.
Bohula is an assistant professor of medicine at Harvard Medical School, Brigham & Women’s Hospital and an investigator with the TIMI Study Group.
“Together with data from genetic studies of PCSK9 variants and other PCSK9 inhibitor outcomes studies, our findings suggest that long-term lowering with PCSK9 inhibitors can help to improve cardiovascular morbidity and potentially mortality over time,” she said.